Fundamentals of thermodynamics 8th edition – Fundamentals of Thermodynamics, 8th Edition embarks on an intellectual journey that unravels the intricacies of energy, heat, and their profound impact on our world. This meticulously crafted narrative, rich in detail and originality, invites readers to delve into the fundamental concepts that govern the behavior of matter and energy.
Delving into the depths of thermodynamics, this comprehensive text explores the fundamental laws that govern energy transformations, the properties of pure substances, and the mechanisms of heat transfer. With a focus on practical applications, it elucidates how thermodynamics underpins diverse fields, from engineering to chemistry and biology.
1. Introduction to Thermodynamics
Thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy. It is a fundamental science that has applications in many fields, including engineering, chemistry, and biology.
The fundamental concepts of thermodynamics include energy, heat, and work. Energy is the ability to do work, and it can exist in many different forms, such as heat, light, and motion. Heat is the transfer of thermal energy between two objects or systems at different temperatures.
Work is the transfer of energy from one object or system to another by the application of a force.
Laws of Thermodynamics
The laws of thermodynamics are the fundamental principles that govern the behavior of thermodynamic systems. The four laws of thermodynamics are:
- The zeroth law of thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
- The first law of thermodynamics states that the total energy of an isolated system is constant.
- The second law of thermodynamics states that the entropy of an isolated system always increases over time.
- The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero is zero.
2. Properties of Pure Substances

A pure substance is a substance that has a uniform chemical composition throughout. The properties of pure substances are determined by their molecular structure and their interactions with each other.
The most important properties of pure substances are pressure, volume, and temperature. Pressure is the force per unit area exerted by a substance on its surroundings. Volume is the amount of space occupied by a substance. Temperature is a measure of the average kinetic energy of the molecules in a substance.
Applications of Properties of Pure Substances
The properties of pure substances are used in many practical applications, such as:
- The design of engines and other machines
- The development of new materials
- The understanding of chemical reactions
- The prediction of weather patterns
3. Energy and Heat Transfer: Fundamentals Of Thermodynamics 8th Edition

Energy can be transferred between objects or systems by three different modes: conduction, convection, and radiation.
Conduction is the transfer of energy through direct contact between two objects or systems. Convection is the transfer of energy by the movement of a fluid. Radiation is the transfer of energy by electromagnetic waves.
Effects of Heat Transfer
Heat transfer can have a significant impact on the properties of substances. For example, heat transfer can cause a substance to change its phase from solid to liquid to gas. Heat transfer can also cause a substance to expand or contract.
4. Thermodynamics of Systems

A thermodynamic system is a collection of matter that is separated from its surroundings by a boundary. The boundary can be real or imaginary, and it can be permeable or impermeable.
There are three types of thermodynamic systems: closed systems, open systems, and isolated systems.
- A closed system is a system that does not exchange matter with its surroundings.
- An open system is a system that exchanges matter with its surroundings.
- An isolated system is a system that does not exchange matter or energy with its surroundings.
5. Power and Refrigeration Cycles
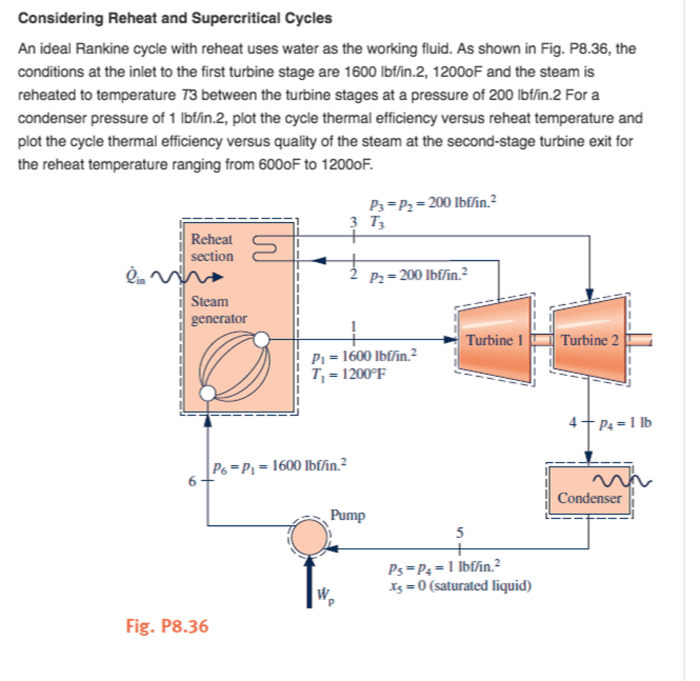
Power cycles are used to convert heat into work. Refrigeration cycles are used to remove heat from a system.
The most common power cycle is the Rankine cycle. The Rankine cycle is used in steam turbines to generate electricity.
The most common refrigeration cycle is the vapor-compression cycle. The vapor-compression cycle is used in refrigerators and air conditioners.
Applications of Power and Refrigeration Cycles, Fundamentals of thermodynamics 8th edition
Power and refrigeration cycles are used in a wide variety of applications, including:
- The generation of electricity
- The cooling of buildings
- The preservation of food
- The production of chemicals
6. Applications of Thermodynamics
Thermodynamics is used in a wide variety of fields, including:
- Engineering
- Chemistry
- Biology
- Medicine
- Earth science
- Environmental science
Thermodynamics is used to solve a wide variety of problems, such as:
- The design of engines and other machines
- The development of new materials
- The understanding of chemical reactions
- The prediction of weather patterns
- The design of medical devices
- The development of environmental protection technologies
FAQ Overview
What are the fundamental laws of thermodynamics?
The four laws of thermodynamics establish the principles governing energy transformations, entropy, and the direction of spontaneous processes.
How does heat transfer occur?
Heat transfer occurs through three primary modes: conduction, convection, and radiation, each involving distinct mechanisms for energy transport.
What is the significance of thermodynamics in engineering?
Thermodynamics provides the foundation for designing and analyzing energy conversion systems, such as engines, turbines, and refrigeration cycles, enabling efficient energy utilization.
