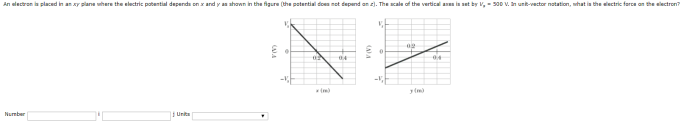
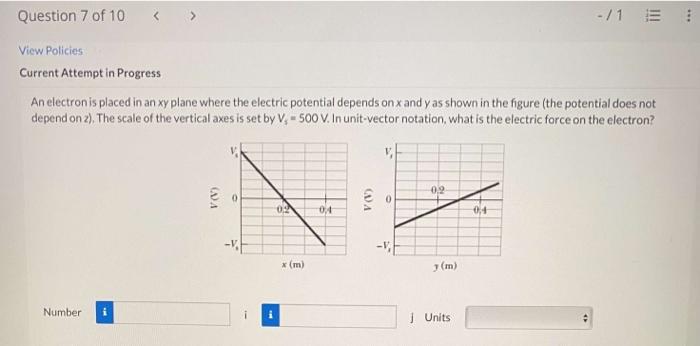
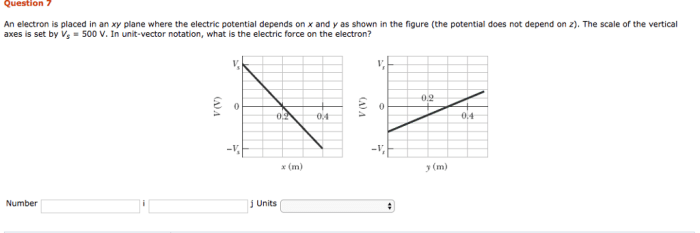
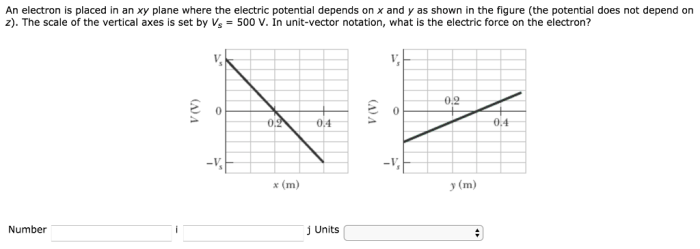
An electron is placed in an xy plane, embarking on a journey that unravels the fundamental properties, motion, and interactions that shape its existence. This exploration delves into the significance of the xy plane in electron positioning, examining the factors that govern its behavior and the diverse applications where its placement plays a pivotal role.
As we delve deeper, we will uncover the intrinsic properties of an electron, including its charge, mass, and spin, and how these attributes influence its movement within the xy plane. The intricate dance of electrons, governed by the laws of electromagnetism, will be brought to light, revealing the factors that determine their trajectory and velocity.
Electron Placement
In the realm of quantum mechanics, electrons are subatomic particles that can be described as existing in a three-dimensional space. The xy plane represents a two-dimensional surface within this space, providing a convenient framework for visualizing and analyzing electron behavior.
The placement of an electron in the xy plane is a fundamental concept in atomic physics. It involves specifying the electron’s position within this two-dimensional surface, typically using Cartesian coordinates (x, y). This placement is crucial for understanding the electron’s wavefunction and its associated quantum states.
Examples of Electron Placement in the xy Plane
- In a hydrogen atom, the electron can occupy specific orbitals, each characterized by a unique wavefunction. These orbitals are often depicted as electron clouds within the xy plane, representing the probability of finding the electron at a given location.
- In a semiconductor device, electrons can be confined to move within a two-dimensional electron gas (2DEG) at the interface between two different materials. The xy plane represents the plane of the 2DEG, and the electron’s placement within this plane determines its energy and transport properties.
- In surface physics, electrons can be confined to move along a surface, forming a two-dimensional electron system (2DES). The xy plane represents the surface, and the electron’s placement within this plane influences its interactions with the surface and other electrons.
Electron Properties
Electrons, fundamental components of atoms, possess unique properties that influence their behavior in the xy plane and contribute to their overall significance in various physical and chemical phenomena.
Charge, An electron is placed in an xy plane
Electrons carry a negative elementary charge, denoted as -e. This property is fundamental to their interactions with other charged particles, including protons and ions. The negative charge of electrons results in electrostatic forces, which play a crucial role in shaping atomic structures and determining chemical bonding.
Mass
Electrons possess a very small mass, approximately 1/1836 times the mass of a proton. Their low mass enables them to exhibit wave-like properties, as described by quantum mechanics, and contributes to their high mobility within atoms and molecules.
Spin
Electrons have an intrinsic property known as spin, which can be visualized as the rotation of the electron around its own axis. Spin is quantized, meaning it can only exist in specific orientations, with two possible spin states: “up” and “down.”
Electron spin plays a significant role in determining the magnetic properties of materials and contributes to the overall behavior of electrons in the xy plane.
The properties of charge, mass, and spin collectively influence the behavior of electrons in the xy plane. These properties determine the electrostatic interactions, wave-like characteristics, and magnetic properties of electrons, shaping their motion and interactions within atoms and molecules.
Electron Motion
Electrons in an xy plane exhibit dynamic motion influenced by various factors. This motion plays a crucial role in shaping the electrical and magnetic properties of materials.
Factors Affecting Electron Motion
The motion of electrons is primarily governed by electric and magnetic fields. Electric fields exert a force on electrons, causing them to accelerate or decelerate. Magnetic fields, on the other hand, induce a force perpendicular to both the magnetic field and the electron’s velocity, resulting in a circular or helical trajectory.
Electron Trajectory and Velocity
The trajectory and velocity of an electron in an xy plane are determined by the strength and direction of the applied electric and magnetic fields. In the absence of magnetic fields, electrons move in straight lines under the influence of electric fields.
When a magnetic field is introduced, the electron’s trajectory becomes curved due to the Lorentz force. The velocity of the electron also changes, increasing or decreasing depending on the field strength and direction.
Significance of Electron Motion
Understanding electron motion is essential for comprehending various electrical and electronic phenomena. It forms the basis for the operation of transistors, diodes, and other semiconductor devices. Additionally, electron motion is crucial in plasma physics, particle accelerators, and many other areas of science and technology.
Electron Interactions: An Electron Is Placed In An Xy Plane
Electrons in the xy plane experience both attractive and repulsive forces due to their electric charges. These interactions play a significant role in determining the behavior and properties of electrons.
Electrons are negatively charged particles, and like charges repel each other. This repulsive force tends to push electrons apart, creating a distance between them. On the other hand, electrons are also attracted to positively charged particles, such as the nuclei of atoms.
This attractive force pulls electrons towards the nucleus, counteracting the repulsive force.
Electron Repulsion
Electron repulsion is the force that acts between electrons due to their like charges. This force is responsible for the distribution of electrons in an atom or molecule. Electrons tend to occupy different energy levels to minimize electron repulsion. The Pauli exclusion principle states that no two electrons can occupy the same quantum state, which further contributes to electron repulsion and the distribution of electrons in different orbitals.
Electron Attraction
Electron attraction is the force that acts between electrons and positively charged particles, such as the nucleus of an atom. This force is responsible for holding electrons in orbit around the nucleus. The attractive force between electrons and the nucleus is stronger than the repulsive force between electrons, which results in electrons being bound to the nucleus.
Examples of Electron Interactions
- Electron repulsion in atoms:Electrons in an atom occupy different energy levels to minimize electron repulsion. This distribution of electrons determines the chemical properties of the atom.
- Electron attraction in molecules:Electrons are attracted to the positively charged nuclei of atoms, forming chemical bonds. The strength of the attractive force between electrons and nuclei determines the stability of the molecule.
- Electron interactions in solids:In solids, electrons are delocalized and can move freely throughout the material. The interactions between electrons in solids determine the electrical and thermal properties of the material.
Electron Applications
The placement of electrons in the xy plane has a profound impact on various applications, particularly in the fields of electronics, optics, and microscopy. The precise control over electron placement enables the manipulation of electrical, optical, and magnetic properties at the nanoscale, leading to the development of advanced devices and technologies.
Electronics
In electronics, electron placement is crucial for the functioning of transistors, the fundamental building blocks of modern computers. By carefully positioning electrons within the semiconductor material, it is possible to control the flow of electrical current, enabling the creation of logic gates and other electronic circuits.
The precise placement of electrons allows for the miniaturization of transistors, leading to the development of more powerful and efficient electronic devices.
Optics
In optics, electron placement plays a vital role in the manipulation of light. By controlling the placement of electrons in materials, it is possible to alter their optical properties, such as refractive index and absorption. This enables the development of optical devices such as lenses, mirrors, and filters that can manipulate light in unprecedented ways.
Electron placement also contributes to the development of optoelectronic devices, which combine electronic and optical functionalities, leading to applications in telecommunications, imaging, and sensing.
Microscopy
In microscopy, electron placement is essential for achieving high-resolution imaging. Electron microscopes utilize a beam of electrons to probe the structure of materials at the atomic level. By controlling the placement of electrons within the beam, it is possible to focus the beam and obtain images with unprecedented detail.
Electron microscopy has revolutionized the field of materials science, allowing researchers to visualize and understand the structure and properties of materials at the nanoscale.
FAQ Corner
What is the significance of the xy plane in electron positioning?
The xy plane provides a convenient reference frame for describing the position and motion of an electron. It allows us to visualize the electron’s movement in two dimensions, simplifying the analysis of its behavior.
How does the charge of an electron influence its motion in the xy plane?
The negative charge of an electron makes it susceptible to electric fields. These fields can accelerate or decelerate the electron, altering its trajectory and velocity within the xy plane.
What is electron repulsion, and how does it affect electron interactions?
Electron repulsion is a force that exists between electrons due to their like charges. This force causes electrons to repel each other, influencing their movement and distribution within the xy plane.