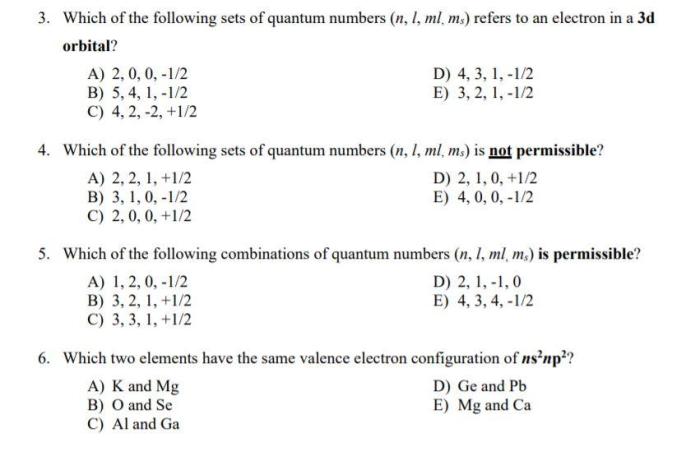
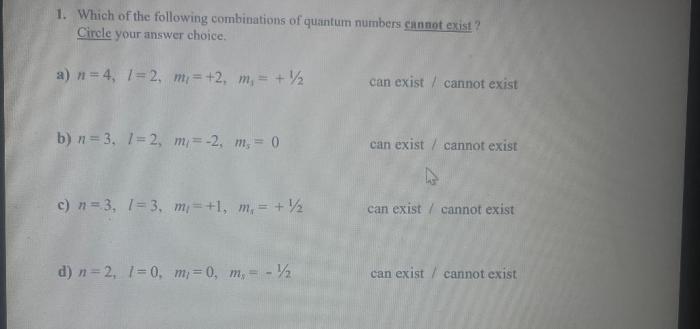
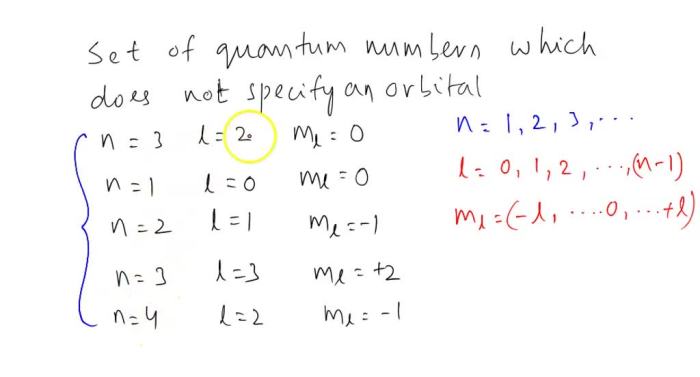
Select the acceptable sets of quantum numbers in an atom. – In the realm of quantum mechanics, the concept of quantum numbers holds paramount importance in deciphering the intricate properties of electrons within atoms. This article delves into the fascinating world of quantum numbers, exploring their significance, rules, and implications in shaping atomic behavior.
Quantum numbers, a quartet of numerical values (n, l, ml, ms), serve as fundamental descriptors of electron characteristics. They govern energy levels, orbital shapes, and electron spin, providing a comprehensive understanding of atomic structure and behavior.
Quantum Numbers and their Significance: Select The Acceptable Sets Of Quantum Numbers In An Atom.
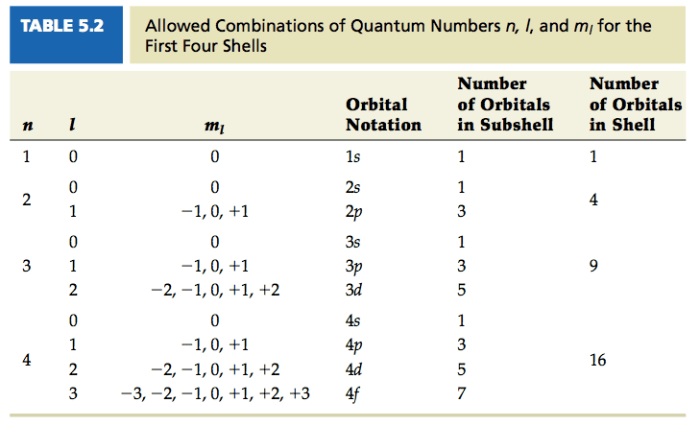
Quantum numbers are a set of four numbers that describe the state of an electron in an atom. They are essential for understanding the behavior of electrons and the properties of atoms.
The four quantum numbers are:
- Principal quantum number (n): Describes the energy level of the electron.
- Azimuthal quantum number (l): Describes the shape of the electron orbital.
- Magnetic quantum number (ml): Describes the orientation of the electron orbital in space.
- Spin quantum number (ms): Describes the spin of the electron.
Quantum numbers play a crucial role in describing the properties of electrons in an atom. They determine the energy of the electron, its angular momentum, its magnetic moment, and its spin. This information is essential for understanding the chemical behavior of atoms and the formation of molecules.
Acceptable Sets of Quantum Numbers
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This means that each electron must have a unique combination of n, l, ml, and ms.
Hund’s rule states that electrons will occupy orbitals with the same energy in such a way as to maximize the total spin of the atom. This means that electrons will first fill orbitals with the same n and l values, and then they will pair up in orbitals with opposite spins.
The Aufbau principle states that electrons will fill orbitals in the order of increasing energy. This means that electrons will first fill the lowest energy orbitals, and then they will move to higher energy orbitals as needed.
These rules can be used to determine the acceptable sets of quantum numbers for electrons in an atom.
Energy Levels and Orbitals, Select the acceptable sets of quantum numbers in an atom.
The energy levels in an atom are determined by the principal quantum number (n). The higher the value of n, the higher the energy of the level.
The shape of the electron orbitals is determined by the azimuthal quantum number (l). The value of l can be 0, 1, 2, …, n-1. The different values of l correspond to different shapes of orbitals, such as s, p, d, and f orbitals.
The orientation of the electron orbitals in space is determined by the magnetic quantum number (ml). The value of ml can be -l, -l+1, …, l-1, l. The different values of ml correspond to different orientations of the orbitals in space.
The spin of the electron is determined by the spin quantum number (ms). The value of ms can be +1/2 or -1/2. The two values of ms correspond to the two possible spins of the electron.
Electron Configurations
The electron configuration of an atom is a description of the arrangement of electrons in the atom’s orbitals. The electron configuration can be written using the following notation:
nxl y
where:
- n is the principal quantum number
- l is the azimuthal quantum number
- x is the number of electrons in the orbital
- y is the number of orbitals with the same n and l values
For example, the electron configuration of helium is 1s 2. This means that helium has two electrons in the 1s orbital.
The electron configuration of an atom can be used to predict the chemical properties of the atom. For example, atoms with similar electron configurations tend to have similar chemical properties.
| Element | Electron Configuration |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
| Lithium | 1s22s1 |
| Beryllium | 1s22s2 |
| Boron | 1s22s22p1 |
| Carbon | 1s22s22p2 |
| Nitrogen | 1s22s22p3 |
| Oxygen | 1s22s22p4 |
| Fluorine | 1s22s22p5 |
| Neon | 1s22s22p6 |
| Sodium | 1s22s22p63s1 |
| Magnesium | 1s22s22p63s2 |
| Aluminum | 1s22s22p63s23p1 |
| Silicon | 1s22s22p63s23p2 |
| Phosphorus | 1s22s22p63s23p3 |
| Sulfur | 1s22s22p63s23p4 |
| Chlorine | 1s22s22p63s23p5 |
| Argon | 1s22s22p63s23p6 |
| Potassium | 1s22s22p63s23p64s1 |
| Calcium | 1s22s22p63s23p64s2 |
Key Questions Answered
What is the significance of quantum numbers?
Quantum numbers provide a comprehensive description of electron properties, including energy levels, orbital shapes, and spin, enabling a detailed understanding of atomic structure and behavior.
How are acceptable sets of quantum numbers determined?
Acceptable sets of quantum numbers adhere to the Pauli exclusion principle, which prohibits electrons from occupying the same quantum state, and Hund’s rule, which favors electron pairing with parallel spins.
What is the Aufbau principle?
The Aufbau principle dictates the sequential filling of atomic orbitals with electrons, starting from the lowest energy levels and proceeding to higher energy levels.